Because you all deserve more
efficiency
qualified-data
feedback
for your pharmacovigilance processes.
efficiency
qualified-data
feedback
azuma hokoku strives to support end-to-end processes for pharmacovigilance. It will offer an API based approach in side effect reporting for practitioner solutions as well as a full SaaS product to support doctors and drug safety responsibles. Stay tuned for more!
Preview:
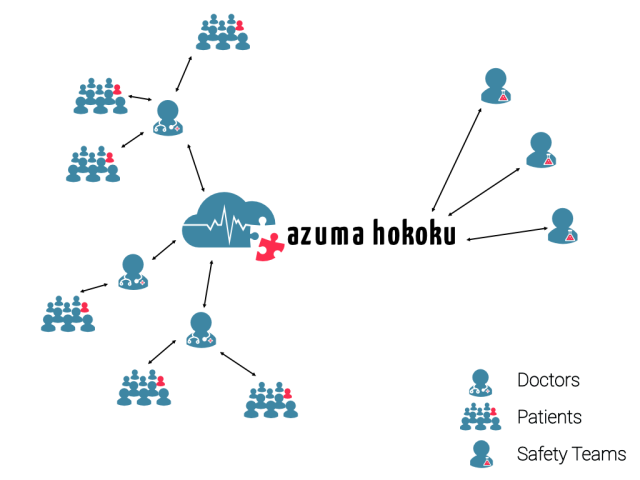
Digitized pharmacovigilance processes
- Doctors can report adverse events, pharma safety teams can manage them
- Exchange of structured data based on ICH and MedDRA standards
- Optimized interfaces for data import and export
- Automations and validation rules where possible to reduce the risk of entry errors
- Adverse event versioning and complete change history, ready for auditing
Your benefits with azuma hokoku
Your safety team will love the efficient workflow. No more media breaks in communicating with side effect reporters, all while improving data quality with more structured data.
One-Stop-Shop
- One integrated SaaS solution
- Synchronous data
- Incoming out outgoing APIs
- Exchange of structured data based on ICH and MedDRA standards
Functionality Out-of-the-Box
- Directly communicate with reporters
- Audit-Ready data
- Secured access via OAuth2 Client credential flows
Easy Integration
- Well-documented and powerful interface
- Customizable GraphQL API calls
- API-only also available for direct integration into your tools